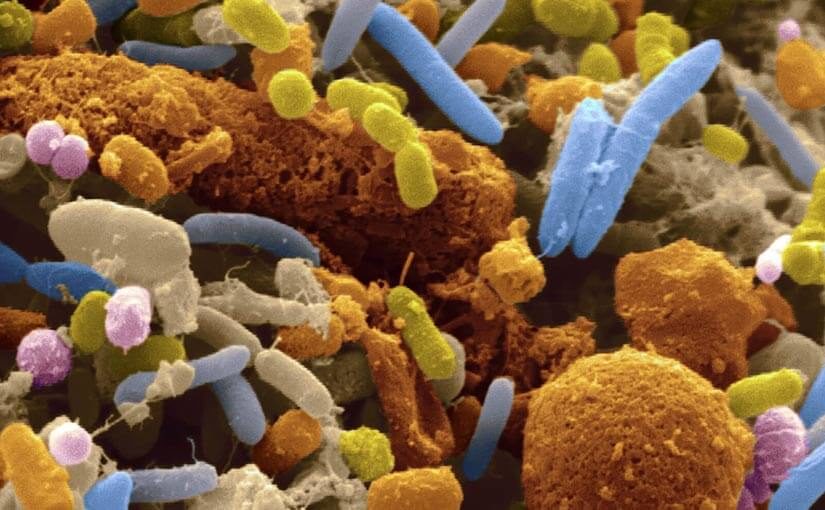
Personalised medicine, the use of targeted drugs and treatments based on an understanding of a person’s genetics, has significantly improved outcomes for cancer patients in recent years. And now research is showing that a person’s gut microbiome (the bacteria and other microbes found in a person’s digestive tract) could play a similarly important role in predicting the effectiveness of a treatment for an individual.
A recent study, published in the journal Nature npj Biofilms and Microbiomes, has demonstrated that gut bacteria can be used to determine whether a cancer drug will work for a certain individual and also if the patient is likely suffer side effects. The study was based on previous evidence that has shown people metabolize drugs in different ways depending on their microbiome.
Although still in the early stages, this research could potentially be the basis for future pathology tests which would help clinicians to better manage cancer treatments and possibly treatments for other diseases, too.
To see whether a person’s microbiome affected how they metabolized cancer drugs, researchers at the Albert Einstein College of Medicine in New York City collected faecal samples from 20 healthy individuals and treated the samples with irinotecan – a chemotherapy drug used to treat colorectal cancer.
When the researchers analysed the treated faecal samples they found that those containing a large amount of an enzyme called beta-glucuronidase, which is produced by gut bacteria, were less able to metabolise the drug. For a patient receiving irinotecan as treatment, this inability to metabolise the drug means they absorb the toxic substance rather than excrete it as waste, and this leads to side effects such as diarrhoea and dehydration.
Commenting on the study, lead researcher A/Professor Libusha Kelly explained the impact of side effects on patients: “Patients with colorectal cancer are already quite ill, so giving them a treatment that causes intestinal problems can be very dangerous. At the same time, irinotecan is an important weapon against this type of cancer.”
The research also demonstrated that beta-glucuronidase enzymes in the gut can interact with other more common drugs including ibuprofen and morphine. This interaction can “reactivate” the drugs in the liver, causing patients to absorb higher than intended doses.
According to Emily Balskus, a biochemist at Harvard University, pathology testing could one day be used to screen people’s microbiomes and determine whether a drug will work for them. If a person’s microbiome seems problematic, doctors could prescribe an enzyme inhibitor or put them on a diet that provides the bacteria with an alternate food source, which could stop beta-glucuronidase enzymes from interfering with the metabolism of the drug.
Dr Nick Musgrave, Pathology Awareness Australia ambassador is interested to see where this kind of research leads;
“Year by year we’re learning more about the impact of the microbiome on our health. The news that it impacts the metabolism of certain chemotherapeutic agents is further proof of this. It will be interesting to see if the microbiome also has an impact on other treatments. Of course these are very early days in our understanding of the effects of an individual’s microbiome and how to manage these effects.”